ProActive® Cool'Bright: The Spirit of Provence’s Peppermint

Mint has a long history with humans. Several species of mint have been used since Babylonian times, though the utility-based nomenclature makes it difficult to link them to today’s peppermints. The first traceable use of peppermint was shortly before the 18th century in England, where it was employed for its fresh and spicy flavor. Considered a digestive and an aromatic medicinal plant, peppermint was admitted in the London Pharmacopoeia under the name Mentha piperitis sapore in 1721. French apothecaries considered it good for the stomach and called it the "English mint,” though from a botanical point of view, peppermint is a hybrid of water mint (Mentha aquatica) and spearmint (Mentha spicata).
Peppermint, compared to sweet mints, is particularly fragrant. Its high menthol content provides a strong sensation of freshness. It is the only mint that is officinal, with digestive virtues. It is sought after both for its herbal qualities and for its ability to produce unique essential oils. Peppermint leaves also contain phenolic acids, in particular rosmarinic acid and caffeic acid, two of the strongest antioxidants. In addition, the leaves are rich in flavonoids, which are also known for their strong antioxidative characteristics.
In Provence, peppermint is cultivated the traditional way: on small surfaces and with a lot of manual work. To have a peppermint rich in menthol, one must never perform more than two cuts per year, between the end June and August. The peppermint is cut flush with the plant using a self-loading mower, shortly after the first flowers appear and at dawn. The harvesting time is critical to the quality of peppermint. This is because the leaves reach their full potential with respect to quality shortly before flowering, and flowering has a negative influence on the menthol/menthone balance. Harvesting after three days of full sun has proven to be the optimum arrangement.
ProActive® Cool'Bright is extracted from fresh Provence peppermint leaves using Proscien’s patent-pending technology, and it brings these essential nutrient molecules to skin. With antioxidants concentrated in a skin-pleasant form and a fragrance characteristic of peppermint, ProActive® Cool'Bright has been proven effective in brightening human skin.

ProActive® Cool'Bright works by inhibiting tyrosinase, thus preventing undesired tanning. At the same time, it cools and comforts skin, particularly irradiated skin.
Tyrosinase is a copper‐containing enzyme widely distributed in nature, present in fungi, higher plants, and animals. In fungi and plants, tyrosinase can oxidize a variety of phenolic compounds, leading to the undesirable browning of fruits and vegetables. In humans, tyrosinase catalyzes the initial step (the hydroxylation of L‐tyrosine to L‐3,4‐dihydroxyphenylalanine, aka L‐DOPA) and the rate‐limiting step (the oxidation of L‐DOPA to o‐quinone) of the melanin formation pathway. Melanin pigments are subsequently generated through a complex series of enzymatic and non‐enzymatic reactions involving cyclization of DOPA–quinone and oxidative polymerization of the resultant indoles.
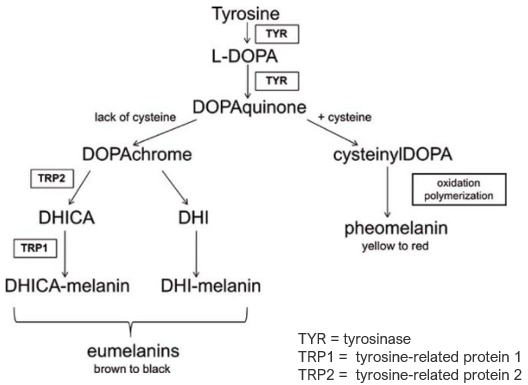
For humans, melanin is important for the prevention of UV damage to skin, hair, and eyes. However, excessive quantities of melanin can lead to neurodegenerative disorders. Abnormal skin pigmentation can also be an aesthetic problem; in some countries, a fair complexion is considered a sign of social distinction.
To effectively treat hyperpigmentation disorders, many have investigated the lightening capabilities of a variety of chemicals. Because tyrosinase catalyzes the key steps of melanin biosynthesis, most of the strategies for developing skin‐lightening or anti‐food‐browning agents are based on the inhibition of tyrosinase activities. Common synthetic tyrosinase inhibitors—such as hydroquinone, ascorbic acid derivatives, kojic acid, or arbutin—are often used to treat dermatological disorders related to melanin and are widely present in cosmetics for their bleaching effect. They have also been associated with toxicity and numerous other side effects. Alternative, more friendly tyrosinase inhibitors are therefore very desirable.
We have demonstrated the effectiveness of ProActive® Cool’Bright in inhibiting tyrosinase activities using a tyrosinase assay. In this assay, DOPA oxidase (diphenolase) activity was spectrophotometrically probed using L‐DOPA as the substrate, and the increase in absorbance at 475 nm due to DOPA chrome formation was recorded. In this test, 20 μl of 5 mM L‐DOPA, 150 μl of 20 mM sodium phosphate buffer (pH 6.8), and 10 μl of the test sample (ProActive® Cool'Bright diluted in ethanol to desired concentrations) were added to a 96‐well plate and incubated at 25 °C for 10 mins. Subsequently, 20 μl of tyrosinase (418 U/ml) were added, and the DOPA chrome formation was immediately monitored by measuring the increase in optical density at 475 nm. The test was carried out at a constant temperature of 25 °C. To evaluate the spontaneous oxidation of L‐DOPA, control experiments were performed in the absence of tyrosinase. In the control experiments, the oxidation of L‐DOPA was negligible. The effects of ProActive® Cool'Bright on tyrosinase activity were represented as percent inhibition at the concentration present in the testing specimen. Concentrations of ProActive® Cool'Bright that inhibited 50% of the tyrosinase activity (IC50), under the experimental conditions, were obtained by nonlinear regression analysis of the inhibition plot.
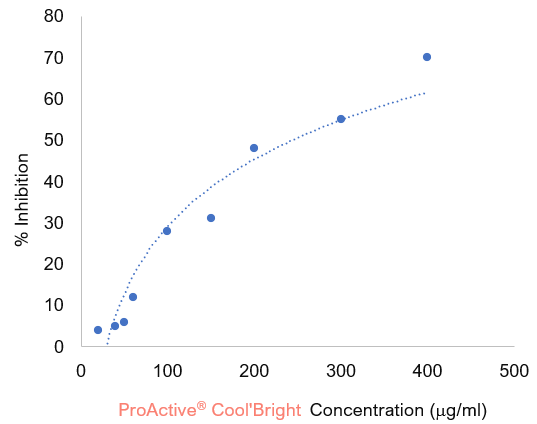
Based on the inhibition plot above, tyrosinase activity was significantly inhibited in a dose-dependent fashion by ProActive® Cool’Bright with IC50 value of 160 μg/ml. The maximal inhibitory effect obtained for ProActive® Cool’Bright was 70% under the experimental conditions, due to limited solubility of ProActive® Cool'Bright in the testing solution.